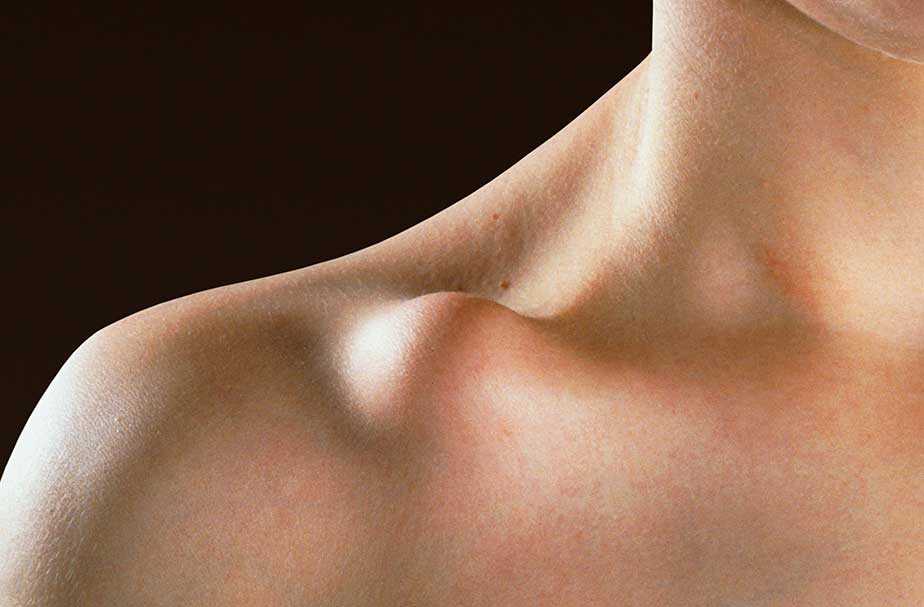
The Sling,
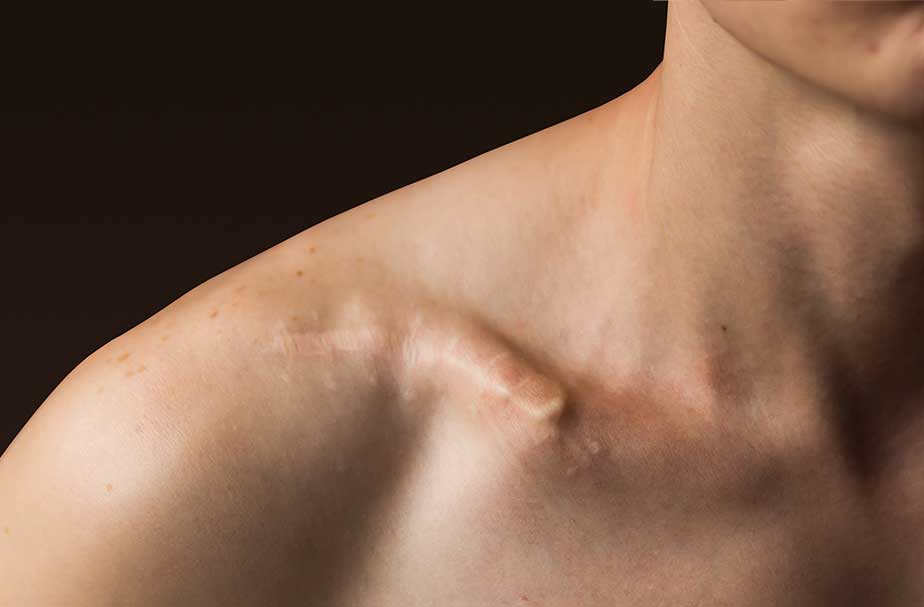
the Plate
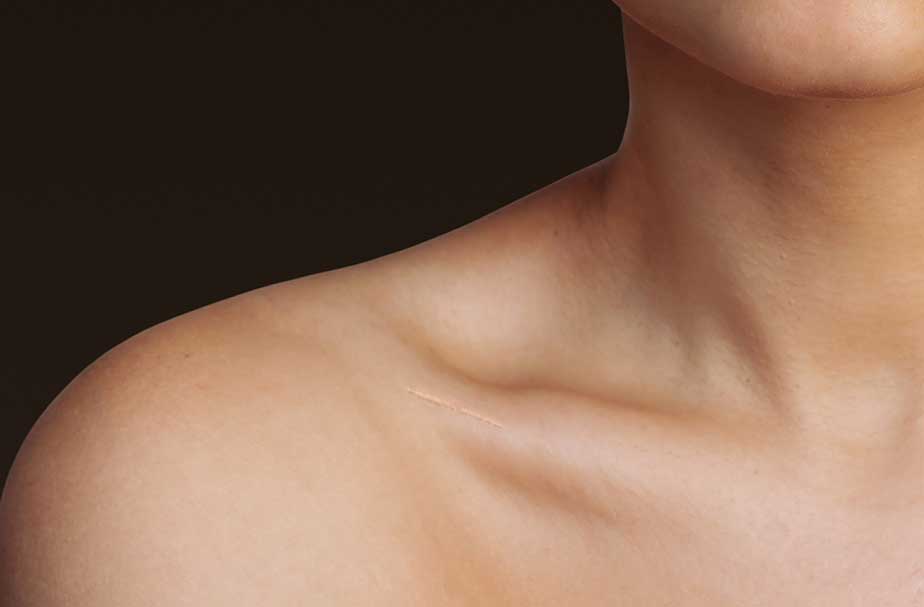
& the Anser
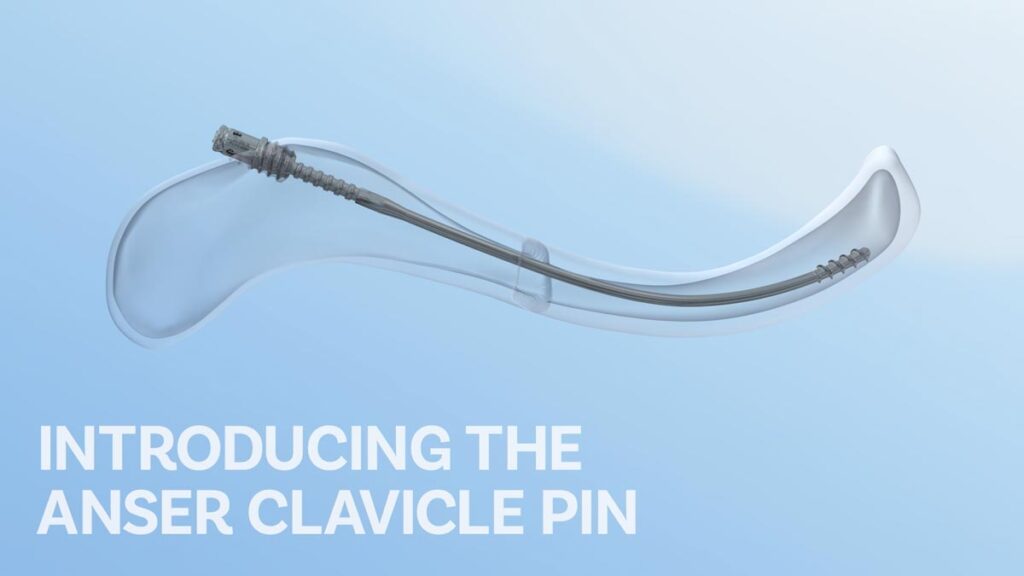
INTRODUCING THE ANSER CLAVICLE PIN
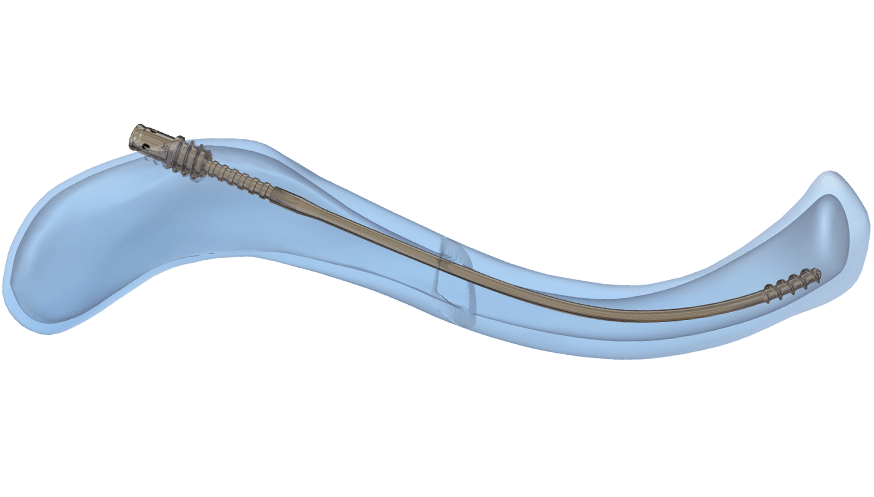
The Anser Clavicle Pin is designed specifically for the treatment of midshaft clavicle fractures. It allows for intramedullary alignment with a flexible design that follows the curvature of the clavicle, providing stability while accommodating micromotion.
- Anchored in both fracture elements to help prevent secondary shortening and migration
- Slim, rotationally-free design allows for torsional forces to dissipate
- Balances flexibility to follow clavicle’s biplanar S-shape, and rigidity to maintain length and reduction
- Quick, simple and minimally invasive procedure
FIXATION THAT’S OPTIMAL
FOR THE CLAVICLE
The Anser Clavicle Pin is thoughtfully designed to address many of the problems associated with legacy interventions.
Plates
- Large incision
- Infection risk
- Prominent scarring
- Sensory nerve damage
- Frequent need for secondary interventions
- Risk of refracture after removal
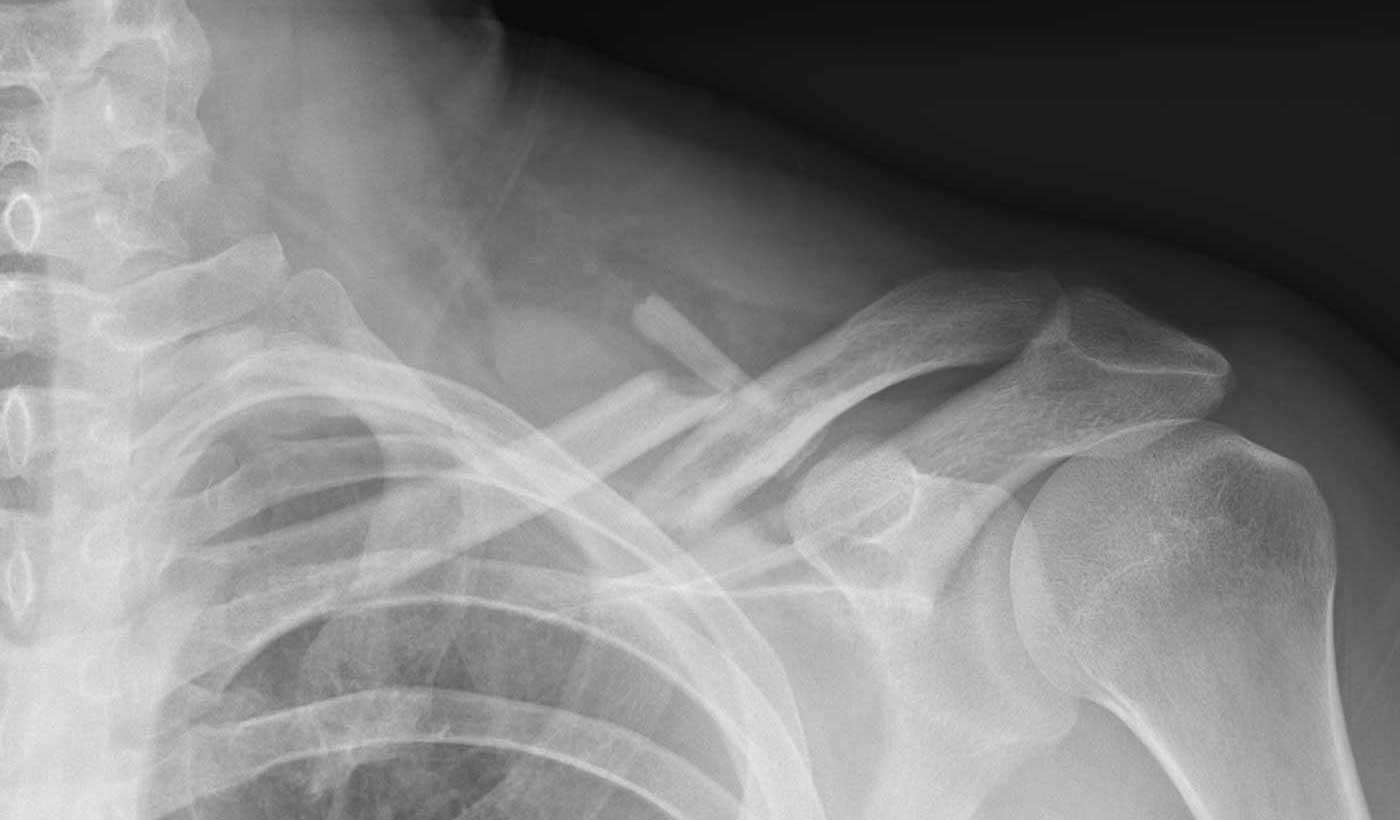
Legacy intramedullary devices
- Prominent, protruding hardware
- Telescoping, migration
- Wound breakdown
- Frequent need for secondary interventions
- Indicated for small subset of fractures
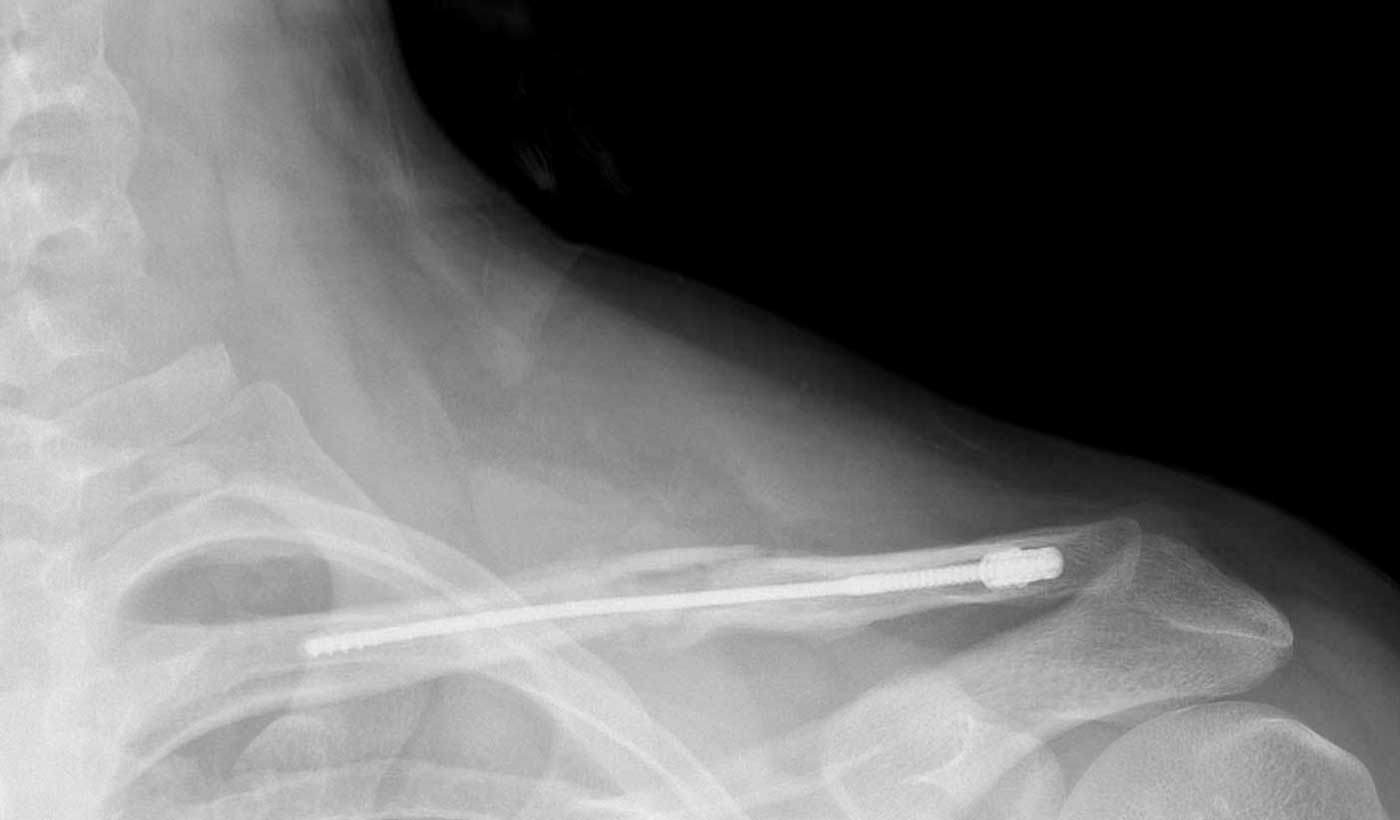
Anser Clavicle Pin
- One size fits all
- Minimally invasive
- Quick and simple procedure
- Intended for all midshaft clavicle fractures
- Reduced need for hardware removal
QUICK,
STRAIGHTFORWARD
PROCEDURE
Inserted from posterior through a small incision, the Anser Clavicle Pin improves aesthetics, while minimizing complications and hardware prominence.
MINIMALLY INVASIVE. OPTIMALLY EFFECTIVE.
The Anser Clavicle Pin was made to solve a problem — the lack of optimal treatment options for midshaft clavicle fractures. For many fractures, nonoperative treatment is inadequate. And the drawbacks with plates can offset their benefits. The Anser Clavicle Pin offers the solution:
- Minimally invasive intramedullary design
- Stabilizes the bone while accommodating micromotion and preserving length
- Allows patients to return to normal activities quickly, without compromised motion
- No disfiguring bumps or scars
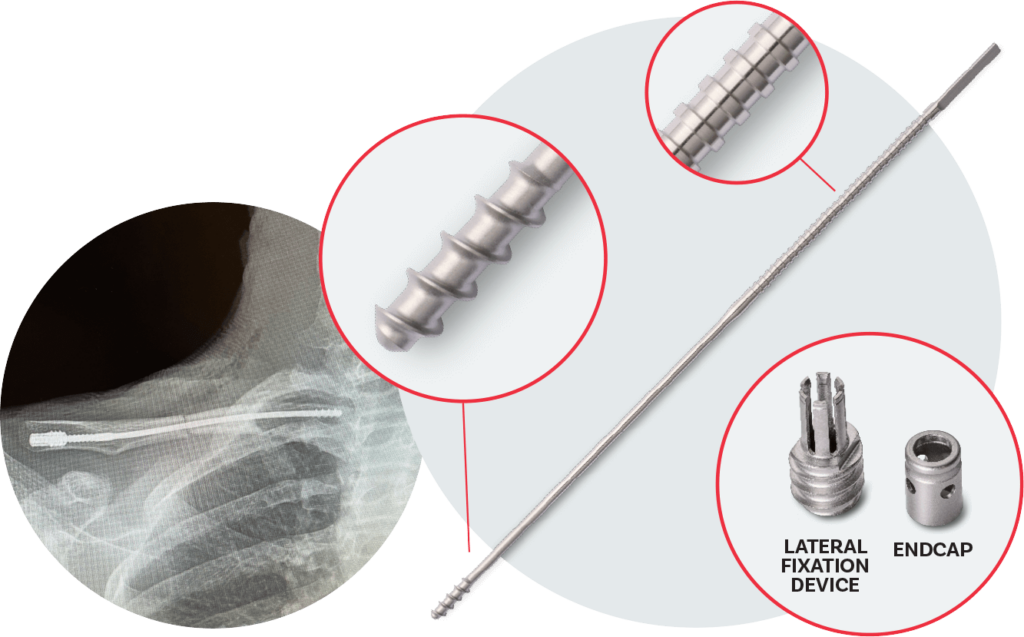
Instrument set
- Pointed 4.0 mm Entry Drill Bit
- Anser Clavicle Pin Tissue Protector
- Anser Clavicle Pin Manual Pin Driver
- Anser Clavicle Pin Cannulated Tap
- Anser Clavicle Pin Lateral Fixation Device Inserter
- Anser Clavicle Pin Endcap Inserter

BAAT Medical BV. is the legal manufacturer of the Anser Clavicle Pin and the Anser Instrument Set. All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Anser Implants Holding BV or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Anser Implants Holding BV. This material is intended for health care professionals. Distribution to any other recipient is prohibited. For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the package insert or contact your local representative. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please email info@anserimplants.com. Check for country product clearances and reference product specific instructions for use. Anser Implants does not practice medicine. This technique was developed in conjunction with health care professionals. This document is intended for surgeons and is not intended for laypersons. Each surgeon should exercise his or her own independent judgment in the diagnosis and treatment of an individual patient, and this information does not purport to replace the comprehensive training surgeons have received. As with all surgical procedures, the technique used in each case will depend on the surgeon’s medical judgment as the best treatment for each patient. Results will vary based on health, age, weight, activity and other variables. Not all patients are candidates for this product and/or procedure. Caution: Federal (USA) law restricts this device to sale by or on the order of a surgeon.
Any surgical intervention is subject to risks and complications. Specific to the Anser Clavicle Pin these could include, but are not limited to, infection, nonunion, malunion, hardware failure, hardware migration, hardware irritation, neuro vascular damage. Rx only. © 2024 Anser Implants. All Rights Reserved.
