COMMON
ISSUES WITH
CONSERVATIVE TREATMENT
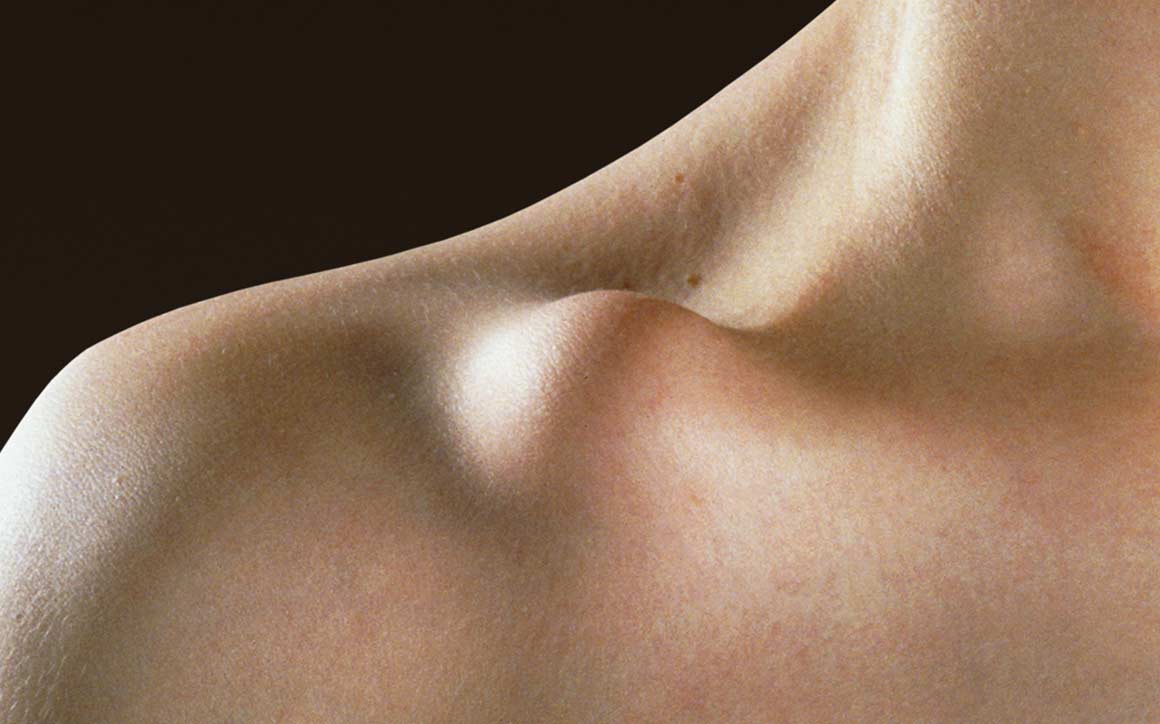
Because of the clavicle’s sigmoid shaped anatomy and muscle insertions, most clavicle fractures are displaced and/or shortened — causing problems with non-unions, persistent posttraumatic symptoms and poor cosmetic outcomes in conservatively treated cases.
SOLVING
PROBLEMS OF
LEGACY
INTERVENTIONS
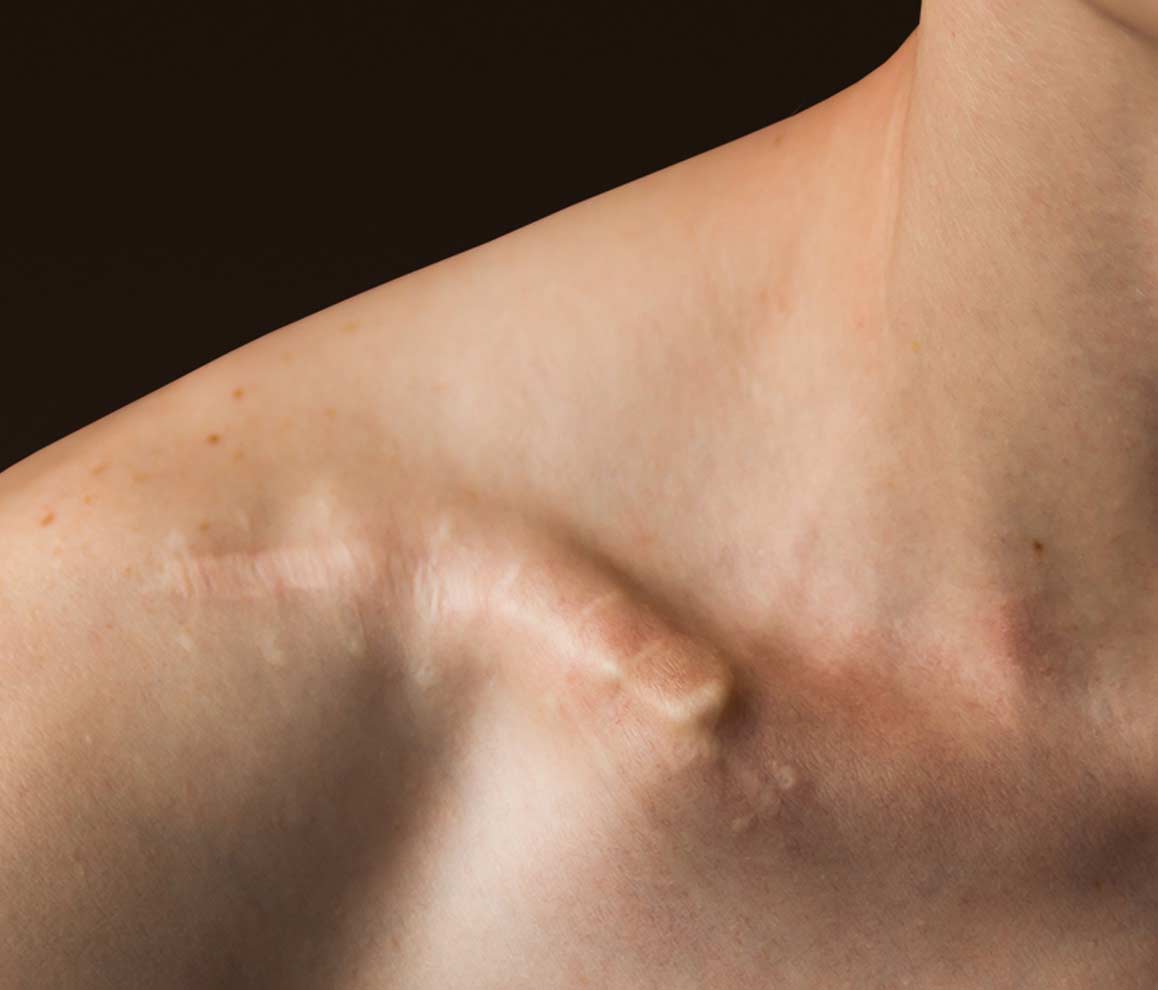
Traditional interventions, such as plates and screws or legacy intramedullary devices, are generally associated with good clinical outcomes. However, these treatments are subject to an array of drawbacks and complications. The Anser Clavicle Pin is designed to address these problems. It reduces and realigns the fractured midshaft clavicle while preserving its length, in a minimally invasive manner.
VIEW ANSER
CLAVICLE PIN
SURGICAL
PROCEDURE

MECHANISM
OF ACTION
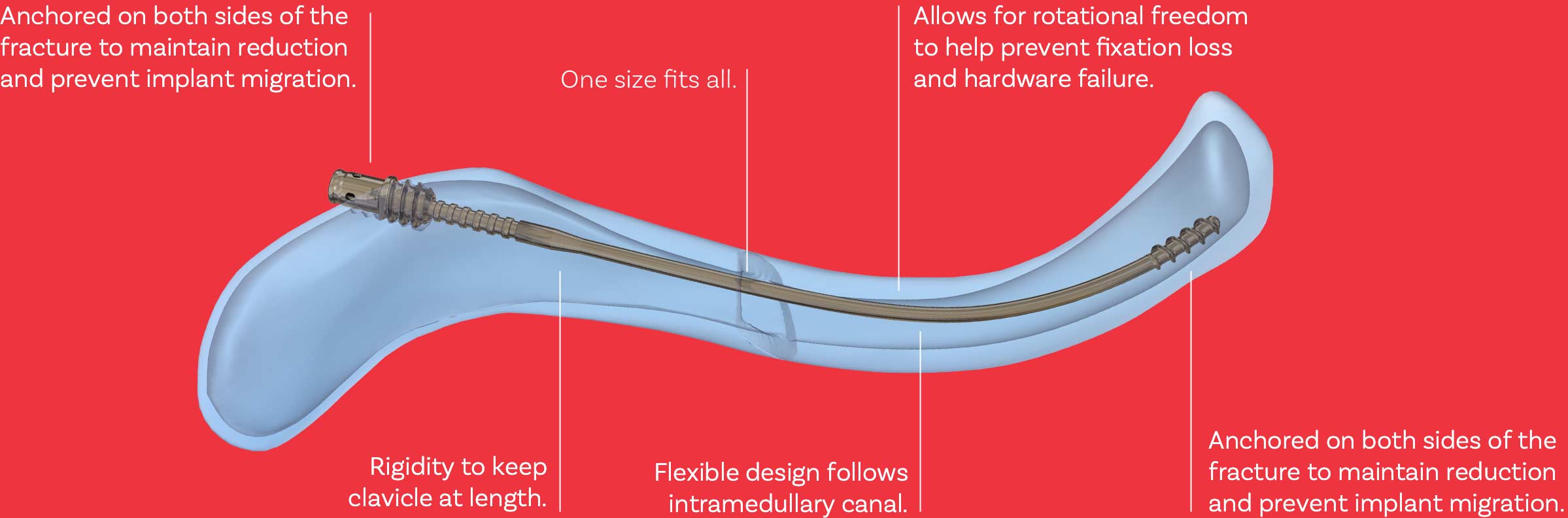
The Anser Clavicle Pin is designed based on the premise that midshaft clavicle fractures do not need absolute stability for proper healing. The fractured clavicle needs to be realigned and kept at length until union occurs; therefore, fixation on both sides of the fracture is required. The design is flexible to follow the contour of the clavicle during insertion and rigid to maintain reduction. The Anser Clavicle Pin is intended as a one-size-fits-all device.
The design of the Anser Clavicle Pin consists of a 2.2 mm flexible pin fabricated from a titanium alloy (Ti-6Al-4V). The Anser Clavicle Pin has a blunt tip which helps prevent perforation. The threaded tip anchors itself against the cortical bone in the medial fracture element.
The Anser Clavicle Pin has a plurality of indentations. These allow the surgeon to adjust the optimal length of the fractured clavicle and pin intra-operatively.
The Anser Lateral Fixation Device device consists of a threaded exterior and six resilient legs. The Anser Lateral Fixation Device anchors itself into the cortical bone of the lateral fracture element by means of the coarse thread. Simultaneously, the resilient legs position themselves around the Anser Clavicle Pin in one of the previously mentioned indentations. Once the optimal length and alignment of the clavicle has been obtained the construct is secured with the Anser End Cap. The Anser Clavicle Pin is cut to length.
During rehabilitation and movement of the arm and shoulder, the clavicle rotates around its longitudinal axis. When fractured, the fracture elements can rotate to a different extent or in opposite directions. To prevent possible friction on the bone-implant interface, loosening of the implant, or hardware failure, the Anser Lateral Fixation Device will allow rotation around the Anser Clavicle Pin while continuing to secure the optimal length. The torsional forces will dissipate within the implant-implant interface.
Besides excellent functional outcomes and patient satisfaction the design of the Anser Clavicle Pin set is expected to reduce the need for hardware removal. This may lead to a reduction in reoperations and associated morbidity. Patients seeking care for their broken collarbone will welcome the shared-decision making conversation when it includes the minimally invasive, optimally effective Anser Clavicle Pin.

RESOURCES
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the package insert or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information,
please email us.
BAAT Medical BV. is the legal manufacturer of the Anser Clavicle Pin and the Anser Instrument Set. All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Anser Implants Holding BV or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Anser Implants Holding BV. This material is intended for health care professionals. Distribution to any other recipient is prohibited. For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the package insert or contact your local representative. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please email info@anserimplants.com. Check for country product clearances and reference product specific instructions for use. Anser Implants does not practice medicine. This technique was developed in conjunction with health care professionals. This document is intended for surgeons and is not intended for laypersons. Each surgeon should exercise his or her own independent judgment in the diagnosis and treatment of an individual patient, and this information does not purport to replace the comprehensive training surgeons have received. As with all surgical procedures, the technique used in each case will depend on the surgeon’s medical judgment as the best treatment for each patient. Results will vary based on health, age, weight, activity and other variables. Not all patients are candidates for this product and/or procedure. Caution: Federal (USA) law restricts this device to sale by or on the order of a surgeon.
Any surgical intervention is subject to risks and complications. Specific to the Anser Clavicle Pin these could include, but are not limited to, infection, nonunion, malunion, hardware failure, hardware migration, hardware irritation, neuro vascular damage. Rx only. © 2024 Anser Implants. All Rights Reserved.
